
A Complete Account of FDA Approvals in 2023
Shots:
- 2023 remained a year of notable approvals by the US FDA. Around 55 drugs were approved, by the US FDA in 2023
- PharmaShots, in an enlightening report, brings a summarized analysis of the approved drugs. The most explored section remains Oncology, Neurology, and Hematology
- For the complete report with analysis, reach out to us at connect@pharmashots.com
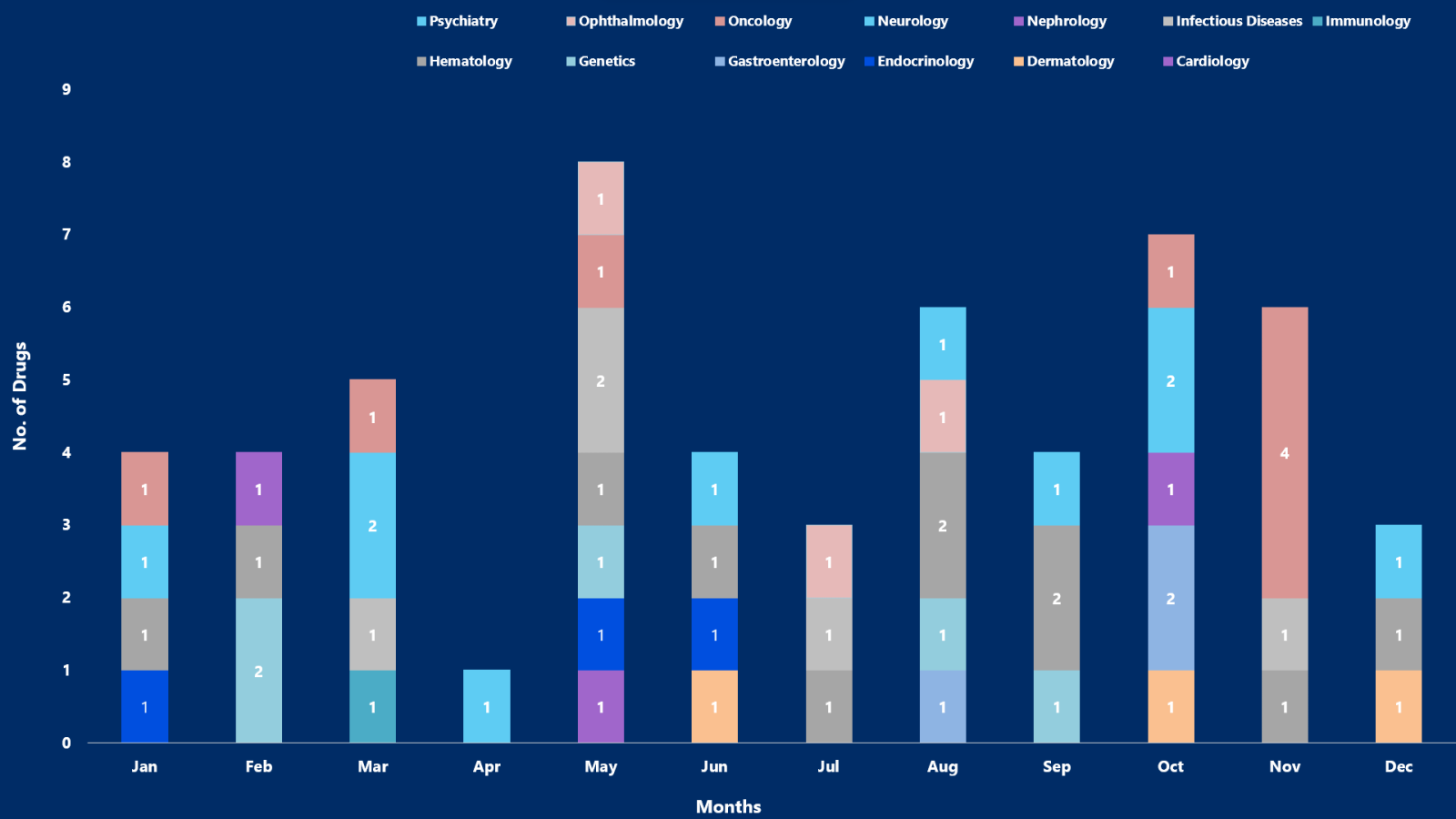
While you were embracing the new year delights on a happy note, PharmaShots was busy compiling a detailed analysis of the list of drugs approved in 2023. With a visualized representation of the approved drugs, we have also incorporated in our report a simplified take on notable approvals with a summarized analysis based on trends, news, and extensive research.
Oncology:
Oncology remains the most explored domain of therapy areas with more than 50 percent of ongoing clinical trials. 2023 witnessed eight noteworthy approvals. Takeda’s Fruzaqla is the first novel chemotherapy-free treatment approved for Metastatic Colorectal Cancer (mCRC). A selective oral inhibitor of VEGFR -1, -2, and -3. Augtyro from BMS, a tyrosine kinase inhibitor is an oral therapy approved for the treatment of adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer.
AstraZeneca’s Truqap, in combination with Faslodex, was approved in the US for the treatment of adults with HR-positive, HER2-negative locally advanced, or metastatic breast cancer with mutations in PIK3CA, AKT1, or PTEN. POSLUMA by Blue Earth is an optimized, high-affinity radio hybrid PSMA-targeted PET imaging agent approved for men with newly diagnosed or suspected occurrence of prostate cancer.
Neurology:
Like Oncology, Neurology too remained relevant throughout 2023. The year saw eight remarkable approvals for several indications. Jointly developed by AstraZeneca and Ionis Pharmaceuticals, Wainua was approved in the US for the treatment of polyneuropathy of hATTR-PN.
Zavzpret by Pfizer and Biohaven was approved by the US FDA for the acute treatment of migraine in adults. Zavzpret is the first and the only calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray for the treatment of migraine in adults with or without aura. Rystiggo by UCB was approved for the treatment of adults with generalized myasthenia gravis.
Qalsody by Biogen was granted accelerated approval for the treatment of ALS in adults with a mutation in the Superoxide dismutase 1 gene.
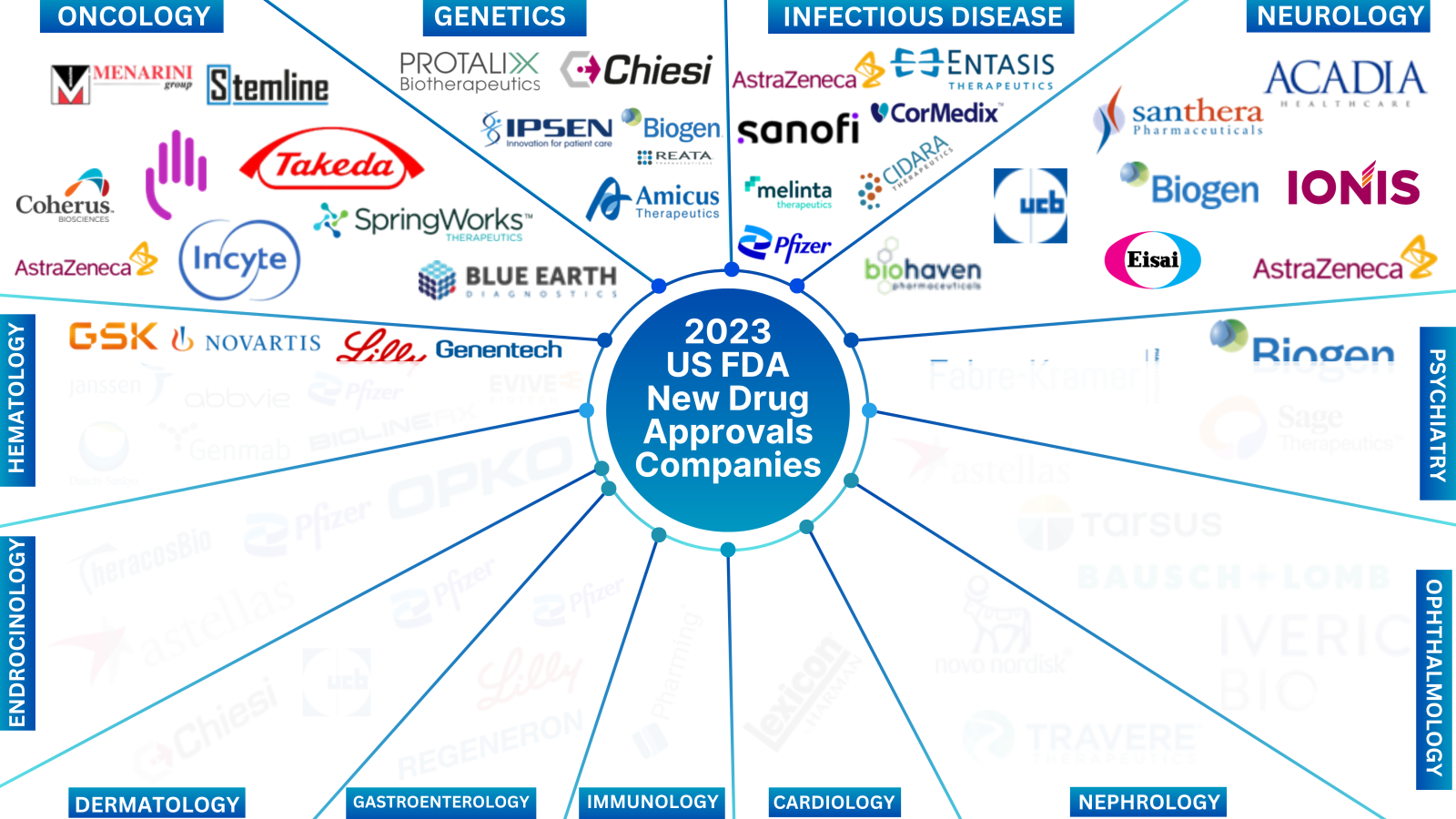
The report only covers the highlights from Oncology and Neurology. For a complete report, reach out to us at connect@pharmashots.com
Closing Note: Propelled by new-age therapeutic interventions, biopharma companies are forging ahead at a momentous pace. Each approved drug symbolizes a small win and holds the potential to slightly transform a particular therapy area. The number of approvals, awareness of diseases, and effort put into tackling them are directly related.

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.














